COVID-19
Immune responses and vaccinesDifferent vaccinesJohn Jacobs, 28.11.2020. |
Many ways exist to develop vaccines and the way vaccines are produced determines their effects on immunity. Since the complexity of the virus immune pathology is only partially understood, it is crucial to understand how vaccines are made, what their mechanisms are and how these are tested. One type of vaccine may be safe and effective due to immunological protection, while the other could potentially aggravate disease.
Attenuated virus and virus for inactivated vaccines is completely dependent on cell culture. Virus vaccines consisting of free proteins or DNA vectors are also mostly produced in cell culture. The new mRNA vaccines are chemically synthesized.
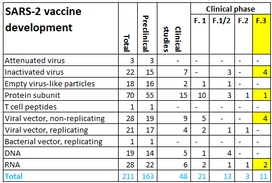
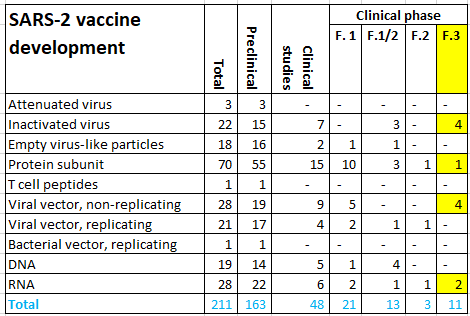
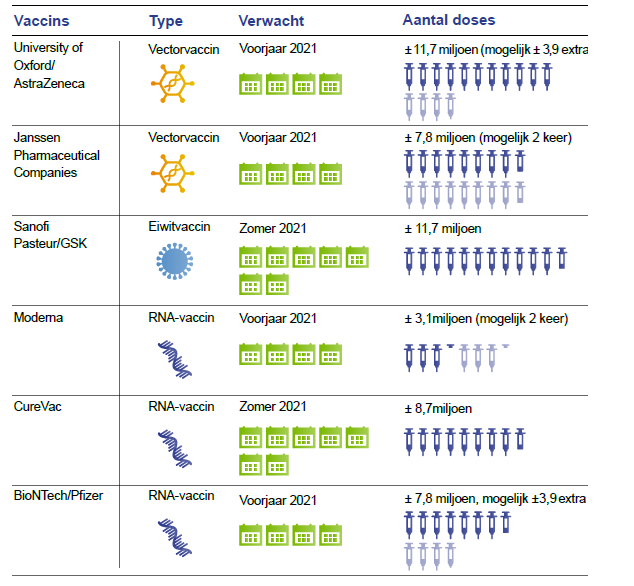
After the preclinical evaluation, vaccines are tested for safety (phase 1) and proof of principle (phase 2) in humans. Next a randomized clinical trial (phase 3 study) is performed to evaluate the efficacy and safety after infection. Subsequently, a vaccine can be submitted to the European Medicines Authority (EMA) and FDA for registration. The World Health Organization (WHO) lists 210 vaccines in development, 163 in preclinical phase and 47 in clinical phase on 12.11.2020. Ten vaccines are in a phase 3 clinical trial (Figure 6). Three of these have been submitted for review to yield EMA registration. EU countries, including the Netherlands have signed contracts with AstraZeneca / Oxford, Janssen Pharmaceutica, Sanofi Pasteur / GSK and BioNTech / Pfizer. Oral agreements have been made with Moderna and CureVac. Negotiations are held with Novavax (Figure 7).
At a higher level, there are three strategies for active vaccines, two of which have major variants. Vaccines can be made from:
Attenuated virus and virus for inactivated vaccines is completely dependent on cell culture. Virus vaccines consisting of free proteins or DNA vectors are also mostly produced in cell culture. The new mRNA vaccines are chemically synthesized.
After the preclinical evaluation, vaccines are tested for safety (phase 1) and proof of principle (phase 2) in humans. Next a randomized clinical trial (phase 3 study) is performed to evaluate the efficacy and safety after infection. Subsequently, a vaccine can be submitted to the European Medicines Authority (EMA) and FDA for registration. The World Health Organization (WHO) lists 210 vaccines in development, 163 in preclinical phase and 47 in clinical phase on 12.11.2020. Ten vaccines are in a phase 3 clinical trial (Figure 6). Three of these have been submitted for review to yield EMA registration. EU countries, including the Netherlands have signed contracts with AstraZeneca / Oxford, Janssen Pharmaceutica, Sanofi Pasteur / GSK and BioNTech / Pfizer. Oral agreements have been made with Moderna and CureVac. Negotiations are held with Novavax (Figure 7).
At a higher level, there are three strategies for active vaccines, two of which have major variants. Vaccines can be made from:
- Attenuated virus
- Viral proteins
- Inactivated virus
- Spike protein
- Viral genen
- DNA-vector with Spike gene
- Messenger RNA (mRNA) of Spike gen
Figure 6. SARS-2 vaccines under developmen
Figure 7. Candidate vaccines for the Netherlands according to the Dutch Health Council.
The Netherlands have 17 million inhabitants and has 3.9% of the total number of vaccines. Because of the same distribution among EU countries other countries have similar doses of vaccines.
The Netherlands have 17 million inhabitants and has 3.9% of the total number of vaccines. Because of the same distribution among EU countries other countries have similar doses of vaccines.
Virus culture
Viruses require living cells to grow. In the early years, animal tissue was cultured, e.g. the tongue cows, but later primary cell culture was introduced. The step forward was to use cancer cells, which have the advantage that their quality and safety is constant. Subsequently secondary cells were introduced that were produced in controlled manner, e.g. PER.C6, in which virtually all putative harmful factors could be excluded. PER.C6 cell cultures are derived from a single cell of an aborted fetus. This cell has been immortalized with the adenovirus E1 gene.
Attenuated viruses
The classic example of this is cow pox, vaccinia. The MMR injection (measles, mumps, and rubella) contains weakened viruses that provide good protection after a mild illness. However, after a SARS-2 infection, immunity is short-lived. For SARS-1, a virus has been made without an envelope (E-gene). That virus could be grown in cell culture. Such an attenuated virus could theoretically recombine with the wild-type virus. A potential recombination in a patient would clearly be undesirable for SARS-2.
Three vaccines with attenuated virus are in the preclinical phase studies.
Three vaccines with attenuated virus are in the preclinical phase studies.
Viruseiwitten
The immune system is triggered by proteins. Proteins can be presented as inactivated virus, empty virus particles, sub-units, protein complexes and peptides.
Attenuated viruses
The classic example of this is cow pox, vaccinia. The MMR injection (measles, mumps, and rubella) contains weakened viruses that provide good protection after a mild illness. However, after a SARS-2 infection, immunity is short-lived. For SARS-1, a virus has been made without an envelope (E-gene). That virus could be grown in cell culture. Such an attenuated virus could theoretically recombine with the wild-type virus. A potential recombination in a patient would clearly be undesirable for SARS-2.
Three vaccines with attenuated virus are in the preclinical phase studies.
Three vaccines with attenuated virus are in the preclinical phase studies.
Inactivated virus
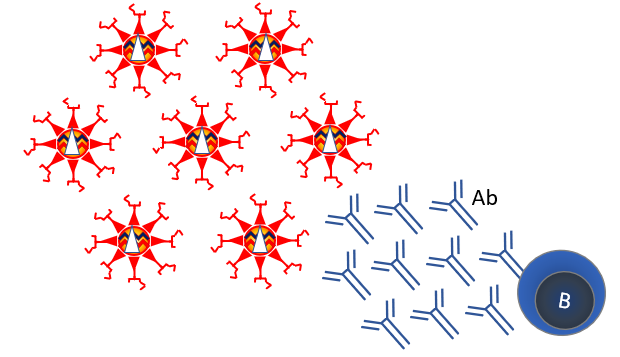
Since the 1950s viruses have been grown in cell culture. Chemical treatment can inactivate this virus, making a safe vaccine. This strategy is used for many vaccines, such as the polio vaccine. Inactivated vaccines mainly elicit good antibody responses (Figure 5). Traditionally, vaccines were primarily evaluated for antibody responses as these are easy to quantify. It is unclear if antibodies protect well against viral infections. An inactivated vaccine against the related SARS-1 led to antibody-mediated exacerbation of the disease in macaques after infection (see Figure 8).
Bharat Biotech (India) and the Chinese producers Sinovac and Sinopharm (2 vaccines) have inactivated vaccines in phase 3 clinical studies. The virus for these vaccines is grown on Vero cells, a cell line derived from green meerkat kidney cells. After cultivation, this virus is inactivated. Nearly 1 million Chinese have received the Sinopharm vaccine. Registration in China differs from randomized and controlled studies (RCTs) in the west. A problem for this study is that the SARS-2 virus hardly infect people in China because of the eradication of the virus in East-Asia. Sinovac tests its vaccines in phase 3 studies in Chile, Indonesia and Turkey.
The Bharat vaccine tests two different adjuvants, the standard variant for Th2 responses and another that may stimulate Th1 responses. Bharat is testing its BBV152B vaccine in India.
The EU and the Netherlands have no plans to purchase inactivated COVID-19 vaccines.
Bharat Biotech (India) and the Chinese producers Sinovac and Sinopharm (2 vaccines) have inactivated vaccines in phase 3 clinical studies. The virus for these vaccines is grown on Vero cells, a cell line derived from green meerkat kidney cells. After cultivation, this virus is inactivated. Nearly 1 million Chinese have received the Sinopharm vaccine. Registration in China differs from randomized and controlled studies (RCTs) in the west. A problem for this study is that the SARS-2 virus hardly infect people in China because of the eradication of the virus in East-Asia. Sinovac tests its vaccines in phase 3 studies in Chile, Indonesia and Turkey.
The Bharat vaccine tests two different adjuvants, the standard variant for Th2 responses and another that may stimulate Th1 responses. Bharat is testing its BBV152B vaccine in India.
The EU and the Netherlands have no plans to purchase inactivated COVID-19 vaccines.
Figure 8. Inactivated virus vaccine
Empty virus particles
Virus-like particles are virus particles without the genetic material (DNA or RNA). These are used for protein folding and shaping in protein vaccines. Thus, the virus-like particles are not derived from coronaviruses, but from other viruses, e.g. plant viruses. The Spike protein is integrated in this structure. Some companies are engaged in early clinical studies of empty virus particles.
Sub-unit vaccins
Recombinant DNA techniques allow cloning and production of a single specific virus gene. This is applied in vaccines against various bacteria, such as pneumococci, Hib and meningococci, and for viruses such as HPV and hepatitis B vaccine.
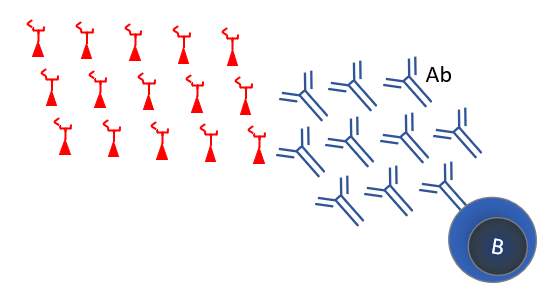
Nearly all SARS-2 vaccines use the Spike (S) protein, because it induces strong neutralizing antibodies (Figure 9). The unnamed Sanofi / GSK vaccine is an adjuvanted recombinant Spike protein. An adjuvant is a substance that stimulates the immune response. This vaccine is still in clinical phase 1/2, comparing different formulations and adjuvants in 11 different combinations. This is one of the vaccines that the EU and Netherlands have an option for, provided that the clinical phase 3 study gives good results.
Novavax is a protein vaccine that uses a saponine-based matrix to enhance immune response. Saponin adjuvants direct the immune responses to a Th1 / Th2 balance, thus include cellular immunity, while protein vaccines normally only induce a Th2 response with antibodies. This vaccine is in a phase 3 study. The EU and the Netherlands are in negotiation with the manufacturer to purchase this vaccine.
Nearly all SARS-2 vaccines use the Spike (S) protein, because it induces strong neutralizing antibodies (Figure 9). The unnamed Sanofi / GSK vaccine is an adjuvanted recombinant Spike protein. An adjuvant is a substance that stimulates the immune response. This vaccine is still in clinical phase 1/2, comparing different formulations and adjuvants in 11 different combinations. This is one of the vaccines that the EU and Netherlands have an option for, provided that the clinical phase 3 study gives good results.
Novavax is a protein vaccine that uses a saponine-based matrix to enhance immune response. Saponin adjuvants direct the immune responses to a Th1 / Th2 balance, thus include cellular immunity, while protein vaccines normally only induce a Th2 response with antibodies. This vaccine is in a phase 3 study. The EU and the Netherlands are in negotiation with the manufacturer to purchase this vaccine.
Peptides
Peptides are part of a protein and are used by dendritic cells to trigger immune responses in Th and Tc cells (see figure 4). Peptide vaccines research is still at an early stage.
Genes not proteins
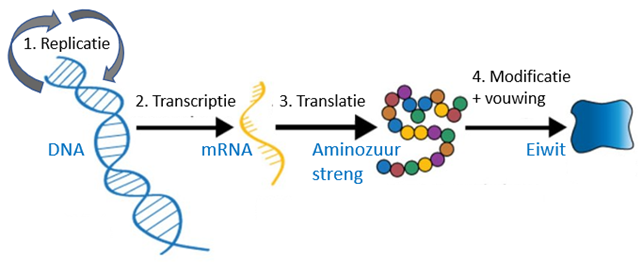
The latest revolution in vaccine technology is that vaccines without proteins, but with genes that are produce these proteins in humans vaccination. These technologies thus to the source in the central dogma of biology (Figure 10). Protein purification and reprocessing is a complex problem. In case of too wild treatment, proteins aggregate, and these aggregates cannot be returned to their original form. Whoever boils or fries an egg can observe this and have a hard time to unboil the egg to its liquid state. Nevertheless, the main reason for this approach is not protein biology but immunology. Protein production within the host is crucial for the development of cellular immunity. Cellular immunity is considered to be most efficient against viruses.
Figure 10. Central dogma of biology.
(1) DNA replication. (2) DNA transcription to mRNA (genetic working copy). (3) mRNA is translated to an amino acid strand. (4) All kinds of chemical reactions take place on that amino acid strand, such as the formation of cross-links and the addition of phosphate, oligosaccharides, and lipid (fat) groups. During this process proteins are folded. Correct folding of proteins is critical to their function. Small differences can have major consequences. A disease like cystic fibrosis, for example, is mainly the result of incorrect protein folding (due to a small mutation). Protein folding is completely different in mammalian cells than in plants or bacteria. This is another big difference between bacteriology and virology. Viruses use mammalian cells, while bacteria make their own proteins.
(1) DNA replication. (2) DNA transcription to mRNA (genetic working copy). (3) mRNA is translated to an amino acid strand. (4) All kinds of chemical reactions take place on that amino acid strand, such as the formation of cross-links and the addition of phosphate, oligosaccharides, and lipid (fat) groups. During this process proteins are folded. Correct folding of proteins is critical to their function. Small differences can have major consequences. A disease like cystic fibrosis, for example, is mainly the result of incorrect protein folding (due to a small mutation). Protein folding is completely different in mammalian cells than in plants or bacteria. This is another big difference between bacteriology and virology. Viruses use mammalian cells, while bacteria make their own proteins.
Vectors
Vectors do not use proteins but their genetic material as a vaccine. If DNA viruses are used, they could replicate themselves. This might be very desirable in cell culture for production, but not always in the vaccinated person. This dilemma is solved by using two vectors, one that is packed in virus-like packaging and the other that makes the necessary proteins but is not wrapped itself. This is the underlying principle of DNA vectors. The Spike protein from SARS-2 is used in all these vaccines.
Scientists selected adenovirus vectors, because vaccination with these resulted in the strongest response in neutralizing antibodies against viruses such as SARS (Figure 9). The adenovirus contains a number of genes that can cause cancer, especially the E1 and E4. These genes are excluded from the vectors. Adenovirus vectors have been in development for several years for vaccines against tuberculosis, malaria and multiple viruses such as Ebola, Zika, influenza and HIV. These were found to be safe in early clinical studies. However, a clinical study on HIV vaccination was discontinued at the time due to lack of safety. The researchers warned of a possible increased susceptibility to HIV.
As discussed before, the higher antibody titers induced by adenovectors could be an advantage or a disadvantage for the efficacy of this vaccine compared to mRNA vaccines. Clinical research will have to show how good these DNA vectors are.
Scientists selected adenovirus vectors, because vaccination with these resulted in the strongest response in neutralizing antibodies against viruses such as SARS (Figure 9). The adenovirus contains a number of genes that can cause cancer, especially the E1 and E4. These genes are excluded from the vectors. Adenovirus vectors have been in development for several years for vaccines against tuberculosis, malaria and multiple viruses such as Ebola, Zika, influenza and HIV. These were found to be safe in early clinical studies. However, a clinical study on HIV vaccination was discontinued at the time due to lack of safety. The researchers warned of a possible increased susceptibility to HIV.
As discussed before, the higher antibody titers induced by adenovectors could be an advantage or a disadvantage for the efficacy of this vaccine compared to mRNA vaccines. Clinical research will have to show how good these DNA vectors are.
Vector variants.
Most DNA vectors are derived from viruses, but non-replicating vectors in humans. Replication requires an help vector that is not absent in the vaccine. This is the case for all four vaccines in phase 3 studies. Unlike weakened viruses, non-replicating vectors cannot multiply in humans. The presence of different virus vector genes could shape immune responses.
Another large group are the viral vectors that can replicate. Clinically, they resemble attenuated viruses, theoretically less safe than non-replicating vectors. These may, however, produce stronger immune responses by causing a minor infection.
Finally, one company has developed a bacterial vector that multiplies in bacteria.
Most DNA vectors are derived from viruses, but non-replicating vectors in humans. Replication requires an help vector that is not absent in the vaccine. This is the case for all four vaccines in phase 3 studies. Unlike weakened viruses, non-replicating vectors cannot multiply in humans. The presence of different virus vector genes could shape immune responses.
Another large group are the viral vectors that can replicate. Clinically, they resemble attenuated viruses, theoretically less safe than non-replicating vectors. These may, however, produce stronger immune responses by causing a minor infection.
Finally, one company has developed a bacterial vector that multiplies in bacteria.
Vectors in phase 3 studies
All vectors in phase 3 studies are adenovectors. The Russian Sputnik V vaccine that was registered in Russia in August falls into this category. The clinical phase 1 and 2 of this vaccine have been published, but the phase 3 study, investigating effectiveness and safety in people infected with SARS-2, is still ongoing. European and American scientists therefore doubt the safety and effectiveness of this vaccine because of untransparent test procedures.
A second adenovirus vaccine is CanSino from China. This is also not used in the Netherlands.
Oxford and AstraZeneca (ChAdOx1-S) have already completed the phase 3 study together and this vaccine has been under evaluation by the EMA for registration since October 1.
Another adenovector vaccine (Ad26.COV2.S) from the Leiden company Janssen Pharmaceutical Companies was also evaluated in a phase 3 study.
The EU and the Netherlands have both adenovector vaccines of Oxford and Janssen on the list for purchase, subject to approval of the EMA registration.
All vectors in phase 3 studies are adenovectors. The Russian Sputnik V vaccine that was registered in Russia in August falls into this category. The clinical phase 1 and 2 of this vaccine have been published, but the phase 3 study, investigating effectiveness and safety in people infected with SARS-2, is still ongoing. European and American scientists therefore doubt the safety and effectiveness of this vaccine because of untransparent test procedures.
A second adenovirus vaccine is CanSino from China. This is also not used in the Netherlands.
Oxford and AstraZeneca (ChAdOx1-S) have already completed the phase 3 study together and this vaccine has been under evaluation by the EMA for registration since October 1.
Another adenovector vaccine (Ad26.COV2.S) from the Leiden company Janssen Pharmaceutical Companies was also evaluated in a phase 3 study.
The EU and the Netherlands have both adenovector vaccines of Oxford and Janssen on the list for purchase, subject to approval of the EMA registration.
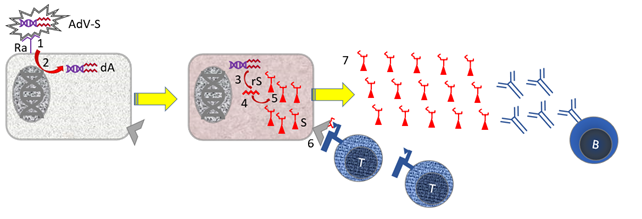
Figure 11. Action of an adenovector vaccine with SARS-2 Spike
Parts: [B] B cell; [AdV-S} Adenovirus vector with Spike gene; [dA} DNA from adenovirus vector including Spike gene [Ra] Receptor for adenovirus vector [rS] RNA encoding Spike protein; [rV] Virus RNA encoding cellular genes; [S] Virus Spike Protein that Binds to the ACE-2 Receptor for Infection. [Tc] cytotoxic T cell; [V] virus. Stepwise Mechanism: [1] The adenovirus vector binds to cells with the receptor for this vector [2] Receptor binding induces membrane fusion and the vector DNA enters the cell's cytoplasm. [3] The vector DNA is multiplied [4] The vector DNA is read for mRNA [5] By translating the virus mRNA, virus proteins are made in the cell. [6] Small pieces of virus proteins (peptides) are presented in the context of MHC I to cytotoxic T cells [Tc]. [7] free virus is produced, against which B cells make antibodies.
Not shown: Steps 6 + 7 are supported by the fact that specialist immune cells (dendritic cells; DC) present small pieces of virus proteins in the context of MHC-II to helper T cells [Th]. See Figure 5 in [Veiligheid voor de huid zonder dierenleed].
Parts: [B] B cell; [AdV-S} Adenovirus vector with Spike gene; [dA} DNA from adenovirus vector including Spike gene [Ra] Receptor for adenovirus vector [rS] RNA encoding Spike protein; [rV] Virus RNA encoding cellular genes; [S] Virus Spike Protein that Binds to the ACE-2 Receptor for Infection. [Tc] cytotoxic T cell; [V] virus. Stepwise Mechanism: [1] The adenovirus vector binds to cells with the receptor for this vector [2] Receptor binding induces membrane fusion and the vector DNA enters the cell's cytoplasm. [3] The vector DNA is multiplied [4] The vector DNA is read for mRNA [5] By translating the virus mRNA, virus proteins are made in the cell. [6] Small pieces of virus proteins (peptides) are presented in the context of MHC I to cytotoxic T cells [Tc]. [7] free virus is produced, against which B cells make antibodies.
Not shown: Steps 6 + 7 are supported by the fact that specialist immune cells (dendritic cells; DC) present small pieces of virus proteins in the context of MHC-II to helper T cells [Th]. See Figure 5 in [Veiligheid voor de huid zonder dierenleed].
DNA vaccines
While vectors contain (viral) proteins, DNA vaccines are devoid of those. The administration of DNA vaccines requires special means such as electroporation. Electroporation uses a small current to shoot DNA into cells. However, these vaccines are all still in early studies.
RNA vaccines
Messenger RNA (mRNA) vaccines are a recent discovery based on chemical technology to make synthetic RNA and lipid membranes. This is a revolution in vaccine technology that allows vaccines to be produced and scaled up very faster, without the need for complex scale-up of cell culture reactors. The mode of action (Figure 11) is very promising from an immunological point of view..
The press releases about the phase 3 trials are very promising for the mRNA vaccines from Moderna (mRNA-1273) and Pfizer/BioNTech (BNT162b2). Both vaccines have also been in the process of registration by the EMA since October 6 and November 16. The EU and the Netherlands have contracts with Pfizer and are talking to Moderna and CureVac (CVnCoV) for purchases of their vaccines. A clinical phase 2 study is now underway for CureVac, in order to find optimal dose.
The press releases about the phase 3 trials are very promising for the mRNA vaccines from Moderna (mRNA-1273) and Pfizer/BioNTech (BNT162b2). Both vaccines have also been in the process of registration by the EMA since October 6 and November 16. The EU and the Netherlands have contracts with Pfizer and are talking to Moderna and CureVac (CVnCoV) for purchases of their vaccines. A clinical phase 2 study is now underway for CureVac, in order to find optimal dose.
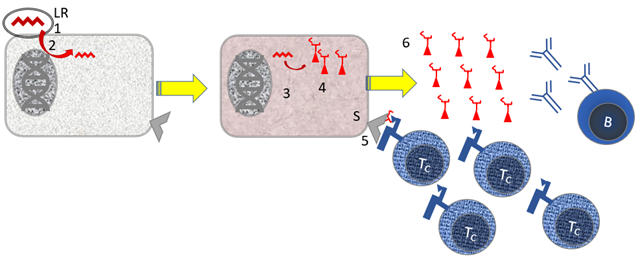
Figure 12. Immunology of mRNA vaccines.
Parts: [B] B cell; [LR} Lipid with (messenger) RNA [Tc] cytotoxic T cell. Stepwise mechanism: [1] The lipids with mRNA bind, possibly non-specifically, to cells [2] The mRNA is taken up into the cytoplasm of the cell. It is striking that the genetic material is not multiplied in the cytoplasm, so that an amplification mechanism is not used. [3] The mRNA for the Spike protein is read in the cell for virus protein. [4] Small pieces of virus proteins (peptides) are presented to cytotoxic T cells in the context of MHC I [Tc]. [5] free virus is produced, against which B cells make antibodies.
Not shown: Steps 4 + 5 are supported by the fact that specialist immune cells (dendritic cells; DC) present small pieces of virus proteins in the context of MHC-II to helper T cells [Th]
Parts: [B] B cell; [LR} Lipid with (messenger) RNA [Tc] cytotoxic T cell. Stepwise mechanism: [1] The lipids with mRNA bind, possibly non-specifically, to cells [2] The mRNA is taken up into the cytoplasm of the cell. It is striking that the genetic material is not multiplied in the cytoplasm, so that an amplification mechanism is not used. [3] The mRNA for the Spike protein is read in the cell for virus protein. [4] Small pieces of virus proteins (peptides) are presented to cytotoxic T cells in the context of MHC I [Tc]. [5] free virus is produced, against which B cells make antibodies.
Not shown: Steps 4 + 5 are supported by the fact that specialist immune cells (dendritic cells; DC) present small pieces of virus proteins in the context of MHC-II to helper T cells [Th]
John Jacobs
28 november 2020
28 november 2020